Sugar cane production is an important industry in tropical regions like Vietnam...
Harvesting

Cane grows very tall in good growing regions, certainly up to 3 metres tall. When ripe it still has some green leaves although most leaves have dried off by then. Where possible the cane is fired before harvesting to remove the dead leaf material and some of the waxy coating. The fire burns at quite high temperatures but is over very quickly so that the cane and its sugar content are not harmed.
In some areas burning is not permitted because of the objection by the local communities of the smoke and carbon specs that are released. However there is no environmental impact, the CO2 released being a very small proportion of the CO2 fixed with photosynthesis during growth and the improved sugar extraction meaning that less cane needs to be grown on fewer acres to satisfy the world's sugar demand.
Harvesting is done either by hand or by machine. Hand cut cane - cane cutting is a hard and dirty job but can employ lots of people in areas where jobs are scarce -- is cut at about ground level, the top green leaves are cropped off and then the stalk is bundled as a whole. Once a complete bundle has been assembled it is removed from the field with a light cart and may then be transferred to a larger vehicle for transport to the mill.
Most machine-cut cane is chopped into short lengths but is otherwise handled in a similar way as hand cut cane. Machines can only be used where land conditions are suitable and the topography is relatively flat. In addition the capital cost of machines and the loss of jobs caused make this solution unsuitable for many sugar estates.
Extraction
The first stage of processing is the extraction of the cane juice. In many factories the cane is crushed in a series of large roller mills: similar to a mangle [wringer] which was used to squeeze the water out of clean washing a century ago. The sweet juice comes gushing out and the cane fibre is carried away for use in the boilers. In other factories a diffuser is used as is described for beet sugar manufacture. Either way the juice is pretty dirty: the soil from the fields, some small fibres and the green extracts from the plant are all mixed in with the sugar.
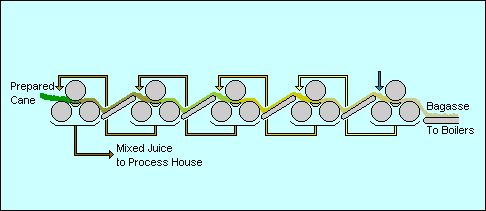
Sugar extraction
A typical mixed juice from extraction will contain perhaps 15% sugar and the residual fibre, called bagasse, will contain 1 to 2% sugar, about 50% moisture and some of the sand and grit from the field as "ash". A typical cane might contain 12 to 14% fibre which, at 50% moisture content gives about 25 to 30 tons of bagasse per 100 tons of cane or 10 tons of sugar.
Liming
The factory can clean up the juice quite easily with slaked lime (a kind of chalk) which settles out a lot of the dirt so that it can be sent back to the fields. This process is known as liming.
The mixed juice from extraction is preheated prior to liming so that the clarification is optimal. The milk of lime, calcium hydroxide or Ca(OH)2, is metered into the juice to hold the required ratio and the limed juice enters a gravitational settling tank: a clarifier. The juice travels through the clarifier at a very low superficial velocity so that the solids settle out and clear juice exits.
The mud from the clarifier still contains valuable sugar so it is filtered on rotary vacuum filters where the residual juice is extracted and the mud can be washed before discharge, producing a sweet water. The juice and the sweet water are returned to the process.
Evaporation
After the liming, the juice is thickened up into a syrup by boiling off the water using steam in a process called evaporation. Sometimes the syrup is cleaned up again but more often it just goes on to the crystal-making step without any more cleaning.
The clear juice has probably only 15% sugar content but saturated sugar liquor, required before crystallisation can occur, is close to 80% sugar content. Evaporation in a steam heated multiple effect evaporator is the best way of approaching the saturated condition.
Boiling/ crystallisation
The syrup is placed into a very large pan for boiling, the last stage. In the pan even more water is boiled off until conditions are right for sugar crystals to grow. Crystal formation is initiated by throwing some crystals into the syrup. Once the crystals have grown the resulting mixture of crystals and mother liquor is spun in centrifuges to separate the two, rather like washing is spin dried. The crystals are then given a final dry with hot air before being stored ready for despatch.

Sugar centrifuges
The mother liquor still contains valuable sugar of course so the crystallisation is repeated several times. However non-sugars inhibit the crystallisation. This is particularly true of other sugars such as glucose and fructose which are the breakdown products of sucrose. Each subsequent step therefore becomes more difficult until one reaches a point where it is no longer viable to continue.
In a raw sugar factory it is normal to conduct three boilings. The first or "A" boiling produces the best sugar which is sent to store. The "B" boiling takes longer and the retention time in the crystalliser is also longer if a reasonable crystal size is to be achieved. Some factories re-melt the B sugar to provide part of the A boiling feedstock, others use the crystals as seed for the A boilings and others mix the B sugar with the A sugar for sale. The "C" boiling takes proportionally longer than the B boiling and considerably longer to crystallise. The sugar is usually used as seed for B boilings and the rest is re-melted.
Additionally, because one cannot get all the sugar out of the juice, there is a sweet by-product made: molasses. This is usually turned into a cattle feed or is sent to a distillery where alcohol is made. This is why rum factories in the Caribbean are always close to sugar cane factories.
Storage
The final raw sugar forms a sticky brown mountain in the store and looks rather like the soft brown sugar found in domestic kitchens. It could be used like that but usually it gets dirty in storage and has a distinctive taste which most people don't want. That is why it is refined when it gets to the country where it will be used.
Affination
The first stage of refining the raw sugar is to soften and then remove the layer of mother liquor surrounding the crystals with a process called "affination". The raw sugar is mixed with a warm, concentrated syrup of slightly higher purity than the syrup layer so that it will not dissolve the crystals, but only the surrounding (brown) liquor. The resulting mixture (‘magma') is centrifuged to separate the crystals from the syrup thus removing the greater part of the impurities from the input sugar and leaving the crystals ready for dissolving before further treatment (carbonatation).
The liquor which results from dissolving the washed crystals contains some colour, fine particles, gums and resins and other non-sugar substances. It is discarded from the process.
Carbonatation
The first stage of processing the sugar-liquor is aimed at removing the solids which make the liquor turbid. Coincidentally some of the colour is removed too. One of the two common processing techniques is known as carbonatation. Carbonatation is achieved by adding milk of lime [calcium hydroxide, Ca(OH)2] to the liquor and bubbling carbon dioxide through the mixture. The gas reacts with the lime to form fine crystalline particles of calcium carbonate which occlude the solids. To obtain a stable flocculation, conditions of the reaction are carefully controlled. The clumps, as they form, collect a lot of the non-sugar substances so that by filtering out the chalk one also takes out these non-sugar substances. Once this is done, the sugar liquor is now ready for decolourisation. The other technique, phosphatation, is chemically similar but uses phosphate rather than carbonate formation. Phosphatation is a slightly more complex process that is achieved by adding phosphoric acid to the liquor after it has been limed in the same way as above.
Decolourisation
There are also two common methods of colour removal from sugar syrup, both relying on absorption techniques with the liquor being pumped through columns of medium. One option open to the refiner is to use granular activated carbon [GAC] which removes most colour but little else. Granular activated carbon is the modern equivalent of "bone char", a carbon granule made from animal bones. Today's carbon is made by specially processing mineral carbon to give a granule which is highly active but also very robust. The carbon is regenerated in a hot kiln where the colour is burnt off from the carbon. The other option is to use an ion exchange resin which removes less colour than GAC but also removes some of the salts present. The resin is regenerated chemically which gives rise to large quantities of unpleasant liquid effluents
The clear, lightly coloured liquor is now ready for crystallisation except that it is a little too dilute for optimum energy consumption in the refinery. It is therefore evaporated prior to going to the crystallisation pan.
Boiling
In the pan even more water is boiled off until conditions are right for sugar crystals to grow. Some sugar dust is added to the liquor to initiate crystal formation. Once the crystals have grown the resulting mixture of crystals and mother liquor is spun in centrifuges to separate the two, rather like washing is spin dried. The crystals are then given a final dry with hot air before being packed and/or stored ready for despatch.
Recovery
The liquor left over from the preparation of white sugar and the washings from the affination stage both contain sugar which can be recovered. They are therefore sent to the recovery house which operates rather like a raw sugar factory, aiming to make a sugar with a quality comparable to the washed raws after the affination stage. As with the other sugar processes, one cannot get all of the sugar out of the liquor and therefore there is a sweet by-product made: refiners' molasses. This is usually turned into a cattle food or is sent to a distillery to make alcohol.
Source: http://www.sucrose.com